
Your message has been sent








The 12-red flags below are very applicable to American Society of Addiction Medicine (ASAM) related consensus and public policy. When viewed through this lens the science and research all falls apart.
(1) When different claims get bundled together.
(2) When ad hominem attacks against dissenters predominate.
(3) When scientists are pressured to toe the party line.
(4) When publishing and peer review in the discipline is cliquish.
(5) When dissenting opinions are excluded from the relevant peer-reviewed literature not because of weak evidence or bad arguments but as part of a strategy to marginalize dissent.
(6) When the actual peer-reviewed literature is misrepresented.
(7) When consensus is declared hurriedly or before it even exists.
(8) When the subject matter seems, by its nature, to resist consensus.
(9) When “scientists say” or “science says” is a common locution.
(10) When it is being used to justify dramatic political or economic policies.
(11) When the “consensus” is maintained by an army of water-carrying journalists who defend it with uncritical and partisan zeal, and seem intent on helping certain scientists with their messaging rather than reporting on the field as objectively as possible.
(12) When we keep being told that there’s a scientific consensus.


Questions about the accuracy and marketing of Laboratory Developed Tests (LDTs) have led to the current debate whether the U.S. Food and Drug Administration (FDA) should regulate a subset of diagnostic tests currently exempted from oversight. Designed to bring clinical tests to market that the costly FDA process would otherwise preclude, such as those for rare diseases, the LDT pathway bypasses Federal regulation and accountability. Questions about the validity of these tests have raised concerns over patient safety and a call for oversight. Among those asking for regulation are Massachusetts Senators Edward J. Markey and Elizabeth Warren.
Opponents of regulation argue the LDT pathway enables new and pioneering tests to be developed quickly and improve patient care. A recent viewpoint piece published in JAMA opposing regulation noted such advances have occurred “in large part because of the nimbleness of relatively small clinical and academic laboratories that can quickly respond to new medical findings and patient needs by rapidly and safely developing and improving laboratory-developed tests.”
But the LDT pathway does not require proof of test validity, that the test is actually testing for what it claims to be testing, and with no FDA oversight a lab can claim any validity it wants in marketing the test. There is no accountability. Proponents of regulation argue that this lack of oversight is a direct threat to patient safety and, as an opposing viewpoint piece in JAMA notes, a “patient’s life or death could hinge on whether a single, unregulated diagnostic test result is meaningful.”
The debate has focused on the reliability and validity of a number of clinical tests currently marketed with unverified claims of accuracy such as those used for prenatal screening and Lyme disease. Notably absent from the discussions are the vast number of Laboratory Developed Tests tests being used for “forensic” drug and alcohol testing with the current FDA draft guidance stating simply:
Numerous “forensic” tests of unknown validity using urine, blood, hair, fingernails breath and saliva have been developed and brought to market as LDTs since the first one was introduced in 2003 when ASAM physician Dr. Gregory Skipper, then Medical Director of the Alabama Physicians Health Program, “convinced the initial lab in the USA, NMS near Philadelphia to start performing EtG testing.”1 With essentially no evidence base Skipper then claimed the alcohol biomarker “appeared to be 100 percent specific” in detecting covert use of alcohol for several days after ingestion based on a study he coauthored that involved a mere 35 forensic psychiatric inpatients in Germany, all male2
 Using an arbitrary cutoff level of 100 ug/L the EtG was marketed as a valid and reliable test and blindly tested on those being monitored by programs not beholden to the strict protocol and procedure dictated by the Mandatory Guidelines for Federal Workplace Drug Testing that most Employee Assistance Programs (EAPs) adopted. In other words, the test was used on those who possessed little power or had their power removed.
Using an arbitrary cutoff level of 100 ug/L the EtG was marketed as a valid and reliable test and blindly tested on those being monitored by programs not beholden to the strict protocol and procedure dictated by the Mandatory Guidelines for Federal Workplace Drug Testing that most Employee Assistance Programs (EAPs) adopted. In other words, the test was used on those who possessed little power or had their power removed.
The test was subsequently found to be so sensitive that it could measure incidental exposure to alcohol in foods, over the counter cold medications, mouthwash3,4, hand sanitizer gel5, and nonalcoholic wine.6 Sauerkraut and bananas have even been shown to cause positive levels.7

Shortly after the EtG debuted, complaints began to accumulate from individuals testing positive who adamantly proclaimed they did not drink. Steadfast in their trust of expert opinion and the claimed accuracy of EtG, the complaints of the accused were largely disregarded by those doing the monitoring. People lost their licenses, jobs, careers, and reputations. Others lost their freedom or had their children taken away. It is unknown how many died by suicide.
There have been multiple lawsuits filed since the introduction of the EtG including a class-action suit, but these were inevitably met with a well-funded and deep legal defense and their “experts.” The labs have taken a “stand your ground” position yielding either dismissals or in favor of the defense. As a new to the market lab with no prior evidence-based research in forensic testing prior to its implementation and use for forensic testing, the proponents of EtG testing had no meaningful opposition in terms of a scientific body of facts and evidence and no credible voice to present it. With the only “experts” in EtG validity being those who introduced and promoted its use there were no counter-forces. Those suffering the consequences of a false-positive test had no recourse. But as the toll of mayhem increased it eventually reached a tipping-point where others began to take notice.

Page from the Talbott Recovery Center list of products containing alcohol that doctors are required to avoid due to interference with EtG testing
In 2006 the Wall Street Journal reported the problems with the EtG to the general public,8 and SAMHSA issued an advisory stating that “legal or disciplinary action based solely on a positive EtG…. is inappropriate and scientifically unsupportable at this time. These tests should currently be considered as potential valuable clinical tools, but their use in forensic settings is premature.”9
Since that time Skipper has served as expert witness in close to 46 administrative hearings 22 criminal 14 custody and 1 Federal class action suit.

But this did not stop the Federation of State Physician Health Programs from using the EtG on physicians being monitored. Instead they instructed doctors to avoid anything potentially containing alcohol including hand sanitizer which a 2011 study found could result in EtG concentrations of almost 2000 ug/L. 10 To continue to justify the use of EtG they added other LDTs as confirmation tests of LDTs such as EtS and PEth– Junk Science to confirm junk science. Nonsensical smoke-and-mirrors antithetical to science and evidence-based medicine.
Since the birth of the EtG a variety of tests have been introduced and marketed as LDTs utilizing nails, blood, hair, breath and urine—all with unknown validity but marketed without constraint. No regulation, oversight or accountability exists.
The newest gadget they are using on doctors is the Cellular Digital Photo Breathalyze which he is promoting in the same manner as the EtG after a study he co-authored with Robert Dupont on just 12 subjects.
Although the current use of these tests is limited to the criminal justice system and professional monitoring programs this may soon change as the American Society of Addiction Medicine is proposing a “new paradigm” of zero-tolerance random widespread drug and alcohol testing. This is outlined in the ASAM White Paper on Drug Testing and described by Robert Dupont in his keynote speech before the Drug and Alcohol Testing Industry Association (DATIA) annual conference in 2012.
The ASAM White paper states drug testing is “vastly underutilized” throughout healthcare and describes the use of drug testing “within the practice of medicine and, beyond that, broadly within American Society.”
As the consequences of a single unregulated “forensic” test result can be grave, far-reaching and even permanent it is critical that these tests be included in the debate on regulation of LDTs.
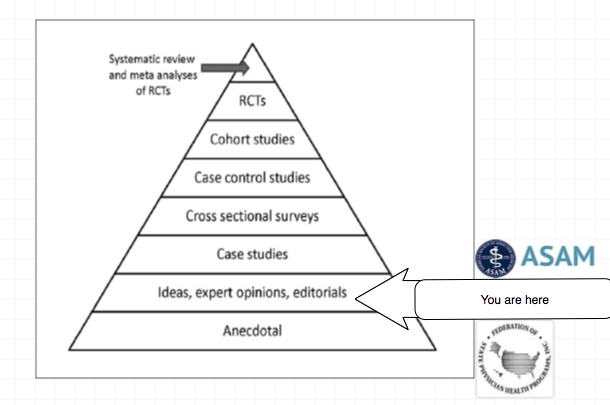
Evidence based medicine is not restricted to randomized trials and meta-analyses. It involves tracking down the best external evidence with which to answer our clinical questions.11
Expert opinion is the lowest level of evidence available in the EBM paradigm.12,13 Fortunately, the scientific method and Cochrane type critical analysis of the available evidence is a tool to help people progress toward the truth despite their susceptibilities to unconscious confirmatory bias or conscious confirmatory distortion .14 Unfortunately, no one has used these tools address they panoply of tests of unknown validity that have already entered the market ; poised to be used on virtually everyone.


It is only a few public policy steps and minor changes in state regulatory statutes before what is described in the ASAM White Paper on Drug Testing comes to fruition. Before we know it the Drug and Alcohol Testing Industries “New Paradigm” as described here by Robert Dupont will be ushered in. From the ASAM white Paper:
“THIS WHITE PAPER ENCOURAGES WIDER AND “SMARTER” USE OF DRUG TESTING WITHIN THE PRACTICE OF MEDICINE AND, BEYOND THAT,BROADLY WITHIN AMERICAN SOCIETY. SMARTER DRUG TESTING MEANS INCREASED USE OF RANDOM TESTING* RATHER THAN THE MORE COMMON SCHEDULED TESTING,* AND IT MEANS TESTING NOT ONLY URINE BUT ALSO OTHER MATRICES SUCH AS BLOOD, ORAL FLUID (SALIVA), HAIR, NAILS, SWEAT AND BREATH WHEN THOSE MATRICES MATCH THE INTENDED ASSESSMENT PROCESS. IN ADDITION, SMARTER TESTING MEANS TESTING BASED UPON CLINICAL INDICATION FOR A BROAD AND ROTATING PANEL OF DRUGS RATHER THAN ONLY TESTING FOR THE TRADITIONAL FIVE-DRUG PANEL.”Backed by the multi-billion dollar drug and alcohol testing, assessment and treatment industry the public policy positions of the American Society of Addiction Medicine (ASAM) have invariably passed. There has been little if any meaningful opposition.
To prevent this future drug testing dystopia, that includes testing schoolchildren, we need to take a step back and analyze the reliability and credibility of the “evidence-base” behind these multiple non-FDA approved (Introduced as Laboratory Developed Tests (LDTs) to bypass FDA approval) “forensic” drug and alcohol tests and testing devices (The alcohol biomarkers EtG, EtS, PEth; SCRAM (Subcutaneous Remote Alcohol Monitoring Bracelet);CDPB (Cellular Digital Photo Breathalyzer); and Hair Testing- Psychemedics, etc.) the ASAM proposes be used on the population at large. These tests include nail, hair, saliva, breath, blood and urine and they plan on utilizing the Medical Profession as a urine collection agency by calling this testing a “medical evaluation” rather than “monitoring” for drug and alcohol use. This change in semantics enables them to bypass the usual forensic drug testing protocol (that includes strict chain-of-custody collection and MRO review) designed to minimize false-positives because the results of erroneous test can be grave and far reaching. According to the ASAM white paper the “clinical” collection of specimens as is good enough as the results of a positive test will result in “treatment” rather than “punishment.”
Amazingly, there has been no Academic review of these tests, let alone a Cochrane type critical analysis. It is essentially untapped territory. In addition there has been no Institute of Medicine type Conflict of Interest Analysis.
And that is why I am asking for help from statisticians, biostatisticians and epidemiologists. The task would entail a review of the literature prior to the introduction of these tests for evidence base of forensic applicability (there essentially is none) and a review of the literature peri-and post marketing of these devices to assess the reliability and credibility of the underlying methodology and ascertain the evidence-base. The goal would be publication in both academic journals and presentation to the general public through media publication with the assistance of investigative journalists and other writers. The goal is to get the truth out about these tests and allow both the medial profession and public at large to awaken to the menace this represents. I can’t pay you but you would be combating injustice, corruption and dishonesty. You would be doing your part in helping the Medical Profession, honest and decent doctors, our country and perhaps our future.
I am looking for a few honest and credible statisticians, biostatisticians or epidemiologists who want to make a difference in the spirit of service and helping others. I can’t pay you but you would be combating injustice, corruption and dishonesty. You would be doing your part in helping the Medical Profession, honest and decent doctors, our country and perhaps our future.
It is only a few public policy steps and minor changes in state regulatory statutes before what is described in the ASAM White Paper on Drug Testing comes to fruition. Before we know it the Drug and Alcohol Testing Industries “New Paradigm” as described here by Robert Dupont will be ushered in as it did with doctors; not with a bang but a whimper. From the ASAM white Paper:
“THIS WHITE PAPER ENCOURAGES WIDER AND “SMARTER” USE OF DRUG TESTING WITHIN THE PRACTICE OF MEDICINE AND, BEYOND THAT,BROADLY WITHIN AMERICAN SOCIETY. SMARTER DRUG TESTING MEANS INCREASED USE OF RANDOM TESTING* RATHER THAN THE MORE COMMON SCHEDULED TESTING,* AND IT MEANS TESTING NOT ONLY URINE BUT ALSO OTHER MATRICES SUCH AS BLOOD, ORAL FLUID (SALIVA), HAIR, NAILS, SWEAT AND BREATH WHEN THOSE MATRICES MATCH THE INTENDED ASSESSMENT PROCESS. IN ADDITION, SMARTER TESTING MEANS TESTING BASED UPON CLINICAL INDICATION FOR A BROAD AND ROTATING PANEL OF DRUGS RATHER THAN ONLY TESTING FOR THE TRADITIONAL FIVE-DRUG PANEL.”To prevent this future drug testing dystopia, that includes testing schoolchildren, we need to take a step back and analyze the reliability and credibility of the “evidence-base” behind these multiple non-FDA approved forensic drug and alcohol tests and testing devices the ASAM proposes be used on the population at large utilizing the Medical Profession as a urine collection agency and bypassing forensic drug testing protocol by calling this “evaluation” and treatment rather than “monitoring” and punishment. New definitions, loopholes, secrecy and subterfuge are the bread and butter of these prohibitionist profiteers.
Amazingly, there has been no Academic review of these tests, let alone a Cochrane type critical analysis. It is essentially untapped territory. In addition there has been no Institute of Medicine type Conflict of Interest Analysis. And that is why I am asking for help from statisticians, biostatisticians and epidemiologists. The task would entail a review of the literature prior to the introduction of these tests for evidence base of forensic applicability (there essentially is none) and a review of the literature peri-and post marketing of these devices to assess the reliability and credibility of the underlying methodology and ascertain the evidence-base. The goal would be publication in both academic journals and presentation to the general public through media publication with the assistance of investigative journalists and other writers. The goal is to get the truth out about these tests and allow both the medial profession and public at large to awaken to the menace this presents to medicine, our society and our future.I am no epidemiologist or statistician but as with pornography I know junk-science when I see it. Almost all of these tests were introduced with little or no evidence-base and, as with most of their endeavors, they did it below board via loopholes and cutting corners.
The overwhelming majority of papers are small, methodologically flawed, non-randomized, non-blinded retrospective studies in that appear to make the data fit the hypothesis. The authors can invariably be linked to those profiting from the tests of the testing process ( the patent holder, doctors associated with the drug testing labs, ASAM or FSPHP, Robert Dupont, Greg Skipper, etc.)
Ethyl Glucuronide (EtG) was introduced in 1999 as a biomarker for alcohol consumption,1 and was subsequently suggested as a tool to monitor health professionals by Dr. Gregory Skipper because of its high sensitivity to ethanol ingestion.2

Described as the “innovator of EtG as an alcohol biomarker,” Skipper and Friedrich Wurst, “convinced” NMS labs in Pennsylvania “to start performing EtG testing in 2002.
The study most often cited as 100% proof that there is 100% accuracy in EtG testing proving alcohol consumption involved a mere 35 forensic psychiatric inpatients in Germany that was published in 2003.3
Shortly thereafter the Physician Health Programs began using it in monitoring doctors and other professional monitoring programs soon followed.
 Laboratory Developed Tests -A Loophole to Avoid FDA Approval and Accountability
Laboratory Developed Tests -A Loophole to Avoid FDA Approval and Accountability
Up until the birth of the EtG tests used for forensic drug and alcohol monitoring had to go through the arduous, expensive and necessary FDA approval process. The LDT pathway was designed to develop simple tests with little risk that have low market potential (i;e. the cost of the normal FDA approval process would prohibit them from coming to market). The LDT pathway was designed to improve patient care and help improve diagnosis and treatment. It was not designed for forensic tests. LDT approval does not require in vivo testing. It is essentially an honor system and to develop an LDT it is not even necessary to prove that the test is actually testing what it is purportedly testing for (validity).
So with little to no evidence base they introduced the EtG, had it developed and marketed as a LDT in collusion with unscrupulous labs, and then began using it on physicians being monitored by State PHPs. This then spread to other monitoring organizations in which there was a large power-differential between those ordering the tests and those being tested (criminal-justice, other professional monitoring programs). These biomarkers have never been used in Federal Drug Testing, SAMHSA approved, DOT, and other organizations where unions or other organizations are present and looking out for the best interests of those being tested.
Another example of how this group removes accountability. There has been essentially no oversight or regulation of LDTs. Although there was a recent push for regulation of these tests the Drug and Alcohol Testing Industry Association lobby made sure that forensic tests would be exempt.They then began publishing “research” on the EtG using the physicians being monitored as subjects. Many of the studies promoting the EtG and other biomarkers can be found in Journals that are linked to organizations that are linked to AA and were organized to educate the medical community.

 These small, methodologically flawed studies amount to little more than opinion pieces but This “evidence-base” is predominantly in biased journals published by biased medical “societies.
The EtG was subsequently found to be so sensitive that it could measure incidental exposure to alcohol in foods, over the counter cold medications, mouthwash4,5, hand sanitizer gel6, nonalcoholic beer7, and nonalcoholic wine.8 Sauerkraut and bananas have even been shown to cause positive EtG levels.9
The United States Substance Abuse and Mental Health Services Administration warned against using a positive EtG as primary or sole evidence of drinking for disciplinary or legal action.10 The Wall Street Journal in 2006 reported the problems with the EtG to the general public.11
These small, methodologically flawed studies amount to little more than opinion pieces but This “evidence-base” is predominantly in biased journals published by biased medical “societies.
The EtG was subsequently found to be so sensitive that it could measure incidental exposure to alcohol in foods, over the counter cold medications, mouthwash4,5, hand sanitizer gel6, nonalcoholic beer7, and nonalcoholic wine.8 Sauerkraut and bananas have even been shown to cause positive EtG levels.9
The United States Substance Abuse and Mental Health Services Administration warned against using a positive EtG as primary or sole evidence of drinking for disciplinary or legal action.10 The Wall Street Journal in 2006 reported the problems with the EtG to the general public.11
 As any rational authority would do, the majority of monitoring agencies abandoned the EtG after these flaws were revealed. The PHPs did not. They continued to use the EtG on doctors uninterruptedly by telling them to avoid any products that could potentially contain alcohol; a ubiquitous substance in the environment. Since that time they have justified and rationalized (EtG)2,12 13 use by sequentially raising cutoff levels from 100 to 250 to 500 to 1000 to 2000 to now unknown and adding other LDTs as “confirmation tests such as Ethyl Sulfate (EtS)14,15 Phosphatidyl-Ethanol ( Peth)16 17 and other devices such as the Subcutaneous Remote Alcohol Monitoring Bracelet (SCRAM) and, their newest device the Cellular Photo Digital Breathalyzer (CPDB) that has recently been launched, just like the EtG
As any rational authority would do, the majority of monitoring agencies abandoned the EtG after these flaws were revealed. The PHPs did not. They continued to use the EtG on doctors uninterruptedly by telling them to avoid any products that could potentially contain alcohol; a ubiquitous substance in the environment. Since that time they have justified and rationalized (EtG)2,12 13 use by sequentially raising cutoff levels from 100 to 250 to 500 to 1000 to 2000 to now unknown and adding other LDTs as “confirmation tests such as Ethyl Sulfate (EtS)14,15 Phosphatidyl-Ethanol ( Peth)16 17 and other devices such as the Subcutaneous Remote Alcohol Monitoring Bracelet (SCRAM) and, their newest device the Cellular Photo Digital Breathalyzer (CPDB) that has recently been launched, just like the EtG  with little to no evidence base other than a pilot study done by Greg Skipper and Robert Dupont.18
A 2013 article published in an ASAM incubated journal Alcoholism: Clinical and Experimental Research promotes the Phosphatidyl-ethanol (PEth ) test to confirm drinking.16 The study was done on physicians being monitored by the Alabama Physician Health Program who tested positive for EtG/EtS alcohol biomarkers. It is co-authored by Robert Dupont, Greg Skipper, and Friedrich Wurst and involved 18 subjects who tested positive for EtG/EtS of whom 7 claimed they did not drink. After finding that 5 of the 7 tested negative for PEth they concluded that “positive PEth testing following positive EtG/EtS results confirms recent drinking. Hard to wrap your head around the science in that one.
with little to no evidence base other than a pilot study done by Greg Skipper and Robert Dupont.18
A 2013 article published in an ASAM incubated journal Alcoholism: Clinical and Experimental Research promotes the Phosphatidyl-ethanol (PEth ) test to confirm drinking.16 The study was done on physicians being monitored by the Alabama Physician Health Program who tested positive for EtG/EtS alcohol biomarkers. It is co-authored by Robert Dupont, Greg Skipper, and Friedrich Wurst and involved 18 subjects who tested positive for EtG/EtS of whom 7 claimed they did not drink. After finding that 5 of the 7 tested negative for PEth they concluded that “positive PEth testing following positive EtG/EtS results confirms recent drinking. Hard to wrap your head around the science in that one.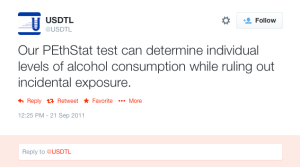 Skipper is also using both Scram ankle bracelets and the CPDB monitoring in pilots in the Human Interventional Motivational Study (HIMS) Program that was developed in 2009 to “identify, treat and, eventually, re-certify airline pilots with substance abuse problems.
Skipper is also using both Scram ankle bracelets and the CPDB monitoring in pilots in the Human Interventional Motivational Study (HIMS) Program that was developed in 2009 to “identify, treat and, eventually, re-certify airline pilots with substance abuse problems.

The Cochrane Collaboration does systematic reviews of the literature using conscientious, explicit, and judicious criteria to in order to produce and disseminate only high quality and evidenced based health care, exclude bias, and enhance transparency. The Cochrane database is a current and evolving database that includes the accuracy of diagnostic tests and is internationally recognized as the standard in evidence based health care. This benchmark for evidence based health care and systematic reviews, records just 5 controlled trials under the topic ethyl glucuronide.8,19-21 These 5 studies represent the only high-quality evidence regarding EtG applying to EtG. Information provided by the five studies suggests the following, and only the following:
Notably, there are no studies that fit Cochrane Criteria, other than non-alcoholic wine, that look at the pharmacokinetics of EtG or EtS in terms of dose-response curves, cut-off levels, specificity drug and food interactions, or modes of ingestion.
SAMHSA notes that there is little research on PEth and that EtG, EtS, and PEth “do not have a strong research base,” and that “it is not known at this time how the test results might be affected by the presence of physical diseases, ethnicity, gender, time, or the use of other drugs. Until considerable more research has occurred, use of these markers should be considered experimental.”
Phosphatidylethanol (PEth), SCRAM, and the yields no data as a test in the Cochrane library.
SAMHSA notes that there is little research on PEth and that EtG, EtS, and PEth “do not have a strong research base,” and that “it is not known at this time how the test results might be affected by the presence of physical diseases, ethnicity, gender, time, or the use of other drugs. Until considerable more research has occurred, use of these markers should be considered experimental.”
Evidence based medicine (EBM) can be defined as the conscientious, explicit, and judicious use of current best evidence in making decisions about the care of individual patients.22
Medical progress and scientific advancement is occurring so fast that the volume of medical literature is expanding at a rate of greater than 7% per year.23
Evidence based medicine is not restricted to randomized trials and meta-analyses. It involves tracking down the best external evidence with which to answer our clinical questions.22
Expert opinion is the lowest level of evidence available in the EBM paradigm.24,25
Fortunately, the scientific method is a tool to help people progress toward the truth despite their susceptibilities to confirmation bias and other errors.26
Unfortunately, due to a confluence of factors (including political) this has not been done. But, unless we want a future as envisioned by Robert Dupont and explained in the the ASAM White Paper on Drug Testing we need to act now. This is not a “New Paradigm” but a “New Inquisition.”




In order to comprehend the current plight of the Medical Profession and the dark clouds that lie ahead it is necessary to understand the history of the “impaired physician movement” and the American Society of Addiction Medicine.
In 1985 the British sociologist G. V. Stimson wrote:
“The impaired physician movement is characterized by a number of evangelical recovered alcoholic and addict physicians, whose recovery has been accompanied by an involvement in medical society and treatment programs. Their ability to make authoritative pronouncements on physician impairment is based on their own claim to insider’s knowledge.”1
The impaired physician movement emphasizes disease and therapy rather than discipline and punishment and believes that addiction is a chronic relapsing brain disease requiring lifelong abstinence and 12-step spiritual recovery. The drug or alcohol abuser or addict is a person lacking adequate internal controls over his or her behavior; for his own protection as well as the protection of society external restraints are required including involuntary treatment.
The American Society of Addiction Medicine can trace its roots to the 1954 founding of the New York City Medical Society on Alcoholism (NYCMSA) by Ruth Fox, M.D whose husband died from alcoholism.
Finding that alcoholics in her psychoanalytic practice did not recover when she used conventional analytic approaches, she taught her patients about alcoholism as a disease and introduced “them to AA meetings held in her living room.”2
A number of physicians in the New York Medical Society were themselves recovering alcoholics who turned to Alcoholics Anonymous for care.3
The society, numbering about 100 members, established itself as a national organization in 1967, the American Medical Society on Alcoholism (AMSA).3
The group promoted the concept of alcoholism as a chronic relapsing disease requiring lifelong spiritual recovery through the 12-steps of AA.
By 1970 membership was nearly 500.2
In 1973 AMSA became a component of the National Council on Alcoholism (NCA), now the National Council on Alcoholism and Drug Dependence (NCADD) in a medical advisory capacity until 1983.
“Abstinence from alcohol is necessary for recovery from the disease of alcoholism” became the first AMSA Position Statement in 1974.2
In 1985 ASAM’s first certification exam was announced. According to Dr. Bean-Bayog, chair of the Credentialing Committee:
“A lot of people in the alcoholism field have long wanted physicians in the field to have a high level of skills and scientific credibility and for this body of knowledge to be accredited.”2And in 1986 662 physicians took the first ASAM Certification Exam.
By 1988 membership was over 2,800 with 1,275 of these physicians “certified” as:
“having demonstrated knowledge and expertise in alcoholism and other drug dependencies commensurate with the standards set forth by the society.”4 “While certification does not certify clinical skill or competence,” the Board explained, “it does identify physicians who have demonstrated knowledge in diagnosis and treatment of alcoholism and other drug dependencies.”5Achieving “recognized board status for chemical dependence” and fellowships in “chemical dependency” are among the five-year objectives identified by the group. These are to come to fruition by “careful discussion, deliberation, and consultation” to “determine its form and structure and how best to bring it about.”5
The formation of ASAM State Chapters begins with California, Florida, Georgia, and Maryland submitting requests.6
In 1988 the AMA House of Delegates votes to admit ASAM to the House. According to ASAM News this “legitimizes the society within the halls of organized medicine.”2
In 1989 the organization changes its name to the American Society of Addiction Medicine (ASAM).2
Since 1990, physicians have been able to list addiction medicine as a self-designated area of practice using the specialty code “ADM.”
By 1993 ASAM has a membership of 3,500 with a total of 2,619 certifications in Addiction Medicine.
The Membership Campaign Task Force sets a goal to double its membership of 3,500 to 7,000 by the year 2000 to assure “the future of treatment for patients with chemicals. It represents a blueprint for establishing addiction medicine as a viable entity.”7
Ninety physicians become Fellows of the American Society of Addiction Medicine (FASAM) in 1996 “to recognize substantial and lasting contributions to the Society and the field of addiction medicine.”8
Among the honorees are Robert DuPont, G. Douglas Talbott, Paul Earley, and Mel Pohl. In addition to at least five consecutive years of membership and certification by the Society, Fellows must have “taken a leadership role in ASAM through committee service, or have been an officer of a state chapter, and they must have made and continue to make significant contributions to the addictions field.”8
The American Board of Addiction Medicine (ABAM) is formed in 2007 as a non-profit 501(C)(6) organization “following conferences of committees appointed by the American Society of Addiction Medicine” to “examine and certify Diplomats.”9
In 2009 National Institute on Drug Abuse (NIDA) Director Nora Volkow, M.D., gives the keynote address at the first ABAM  board certification diploma ceremony.10
board certification diploma ceremony.10
According to an article in Addiction Professional “Board certification is the highest level of practice recognition given to physicians.”
“A Physician membership society such as ASAM, however, cannot confer ‘Board Certification,’ ” but a“ “Medical Board such as ABAM has a separate and distinct purpose and mission: to promote and improve the quality of medical care through establishing and maintaining standards and procedures for credentialing and re-credentialing medical specialties.”
The majority of ASAM physicians meet these requirements by “working in a chemical dependency treatment facility, taking continuing medical education courses in addiction, or participating in research.”11
“In the United States accredited residency programs in addiction exist only for psychiatrists specializing in addiction psychiatry; nonpsychiatrists seeking training in addiction medicine can train in nonaccredited ‘fellowships,’ or can receive training in some ADP programs, only to not be granted a certificate of completion of accredited training.”11
Specialty recognition by the American Board of Medical Specialties, fifty Addiction Medicine Fellowship training programs and a National Center for Physician Training in Addiction Medicine are listed as future initiatives of the ABAM Foundation in 2014.
The American Society of Addiction Medicine’s mission is to “establish addiction medicine as a specialty recognized by professional organizations, governments, physicians, purchasers, and consumers of health care products, and the general public’12
In this they have succeeded.
And in the year 2014 G.V. Stimson’s characterization of the “impaired physician movement” remains as accurate and apt as it was in 1985. But the “number of evangelical recovered alcoholic and addict physicians” has increased dramatically (outnumbering Addiction Psychiatry by 4:1) and their involvement in “ medical society and treatment programs” has been realized and enforced through the state Physician Health Programs and their “PHP-approved’ assessment and treatment centers.
Their “ability to make authoritative pronouncements on physician impairment…based on their own claim to insider’s knowledge” has become public policy and sanctified by Regulatory Medicine -essentially the Word of the Lord.
And the 1953 Alcoholics Anonymous prophecy that “With one arm around the shoulder of religion and the other around the shoulder of medicine, we might change the world” is also coming to pass.
But the world is not changing for the better as that arm around the shoulder of religion has its fingers deep in the pockets of the multi-billion dollar drug and alcohol testing and assessment and treatment industries. And the arm around the shoulder of medicine has its fingers clamped tightly around its throat; a stranglehold in full throttle suffocating the Profession of Medicine with no meaningful opposition I can see.